Description
DESCRIPTION
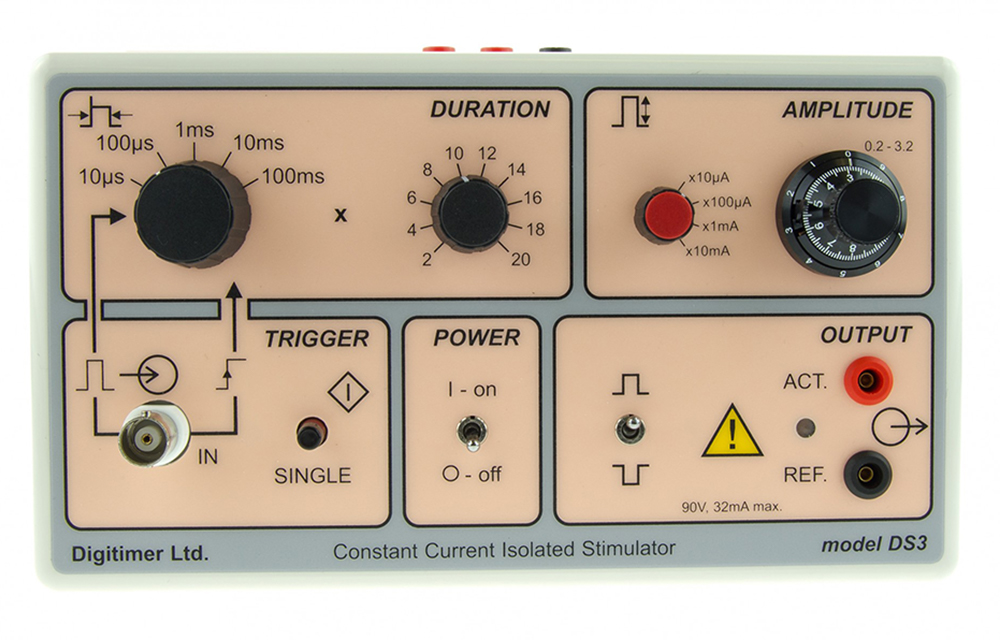
The DS3 provides a precise constant current, isolated stimulus, controllable in pulse duration and amplitude from self-contained batteries. The range of pulse durations available can be varied using a six-position switch and multiplier within the range 20 microseconds to 2 seconds as well as being externally controlled (gated). A three-turn dial on the front panel of the stimulator provides a continuous adjustment of the output amplitude which is indicated on the vernier dial as 0.2 to 3.2. With the four ranges (x10μA; x100μA; x1mA and x10mA), currents from 2μA to 32mA can be selected. The existence of an output pulse is indicated by an LED. The Output is optically isolated from the trigger source ensuring a very low capacitive coupling between trigger source and stimulator to reduce artifacts. The self-contained batteries, without a DC-DC converter, ensure that the stimulator is ultra-quiet and does not induce any high-frequency noise into the recording system.
It is worth noting that battery current is only drawn when the stimulator is delivering an output pulse. This greatly extends the life of the batteries. When constant current stimulators are used to stimulate very high-impedance, high capacitance cells the electrode tip can charge up and stimulation be lost. This is because constant current stimulators have a very high output impedance which will not discharge the cells. The DS3 provides an output discharge circuit which operates (if user enabled) after each stimulus pulse. An internal jumper selects this feature.
The DS3 can be triggered by an external device such as our new DG2A Train/Delay Generator. The DS3 can be fitted into a 19″ rack mounting frame (D121-11) which can hold up to two DS3s.
To find out more about Isolated Current Stimulators like the DS3 you can contact us by calling 01707 328347 or by emailing [email protected]. Our knowledgeable team are more than happy to provide details on any of our products.
GALLERY
DOWNLOADS
DS2A/DS3 Isolated Stimulator
Data sheet
DS3 Application Note
Using two DS3 stimulators in parallel allows stimulation through the same electrodes with different pulse types
DS3 Application Note
Using a DG2A Train/Delay Generator & Two DS3 Isolated Stimulators to Produce a Biphasic Constant Current Stimulus
PUBLICATIONS
The Digitimer DS3 Isolated Constant Current Stimulator has been referenced in over 500 research papers, which can be viewed on Google Scholar. A few of the most highly cited papers published since 2019 are provided below.
Giandomenico, S. L., Mierau, S. B., Gibbons, G. M., Wenger, L. M. D., Masullo, L., Sit, T., … Lancaster, M. A. (2019). Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nature Neuroscience, 22(4), 669–679. https://doi.org/10.1038/s41593-019-0350-2
Kemaladewi, D. U., Bassi, P. S., Erwood, S., Al-Basha, D., Gawlik, K. I., Lindsay, K., … Cohn, R. D. (2019). A mutation-independent approach for muscular dystrophy via upregulation of a modifier gene. Nature, 572(7767), 125–130. https://doi.org/10.1038/s41586-019-1430-x
Krashia, P., Cordella, A., Nobili, A., La Barbera, L., Federici, M., Leuti, A., … Mercuri, N. B. (2019). Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nature Communications. nature.com. https://doi.org/10.1038/s41467-019-11928-w
Figueiredo, C. P., Barros-Aragão, F. G. Q., Neris, R. L. S., Frost, P. S., Soares, C., Souza, I. N. O., … Ferreira, S. T. (2019). Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nature Communications. nature.com. https://doi.org/10.1038/s41467-019-11866-7
Gómez-Suaga, P., Pérez-Nievas, B. G., Glennon, E. B., Lau, D. H. W., Paillusson, S., Mórotz, G. M., … Miller, C. C. J. (2019). The VAPB-PTPIP51 endoplasmic reticulum-mitochondria tethering proteins are present in neuronal synapses and regulate synaptic activity. Acta Neuropathologica Communications. actaneurocomms.biomedcentral …. https://doi.org/10.1186/s40478-019-0688-4
Lippert, R. N., Cremer, A. L., Edwin Thanarajah, S., Korn, C., Jahans-Price, T., Burgeno, L. M., … Backes, H. (2019). Time-dependent assessment of stimulus-evoked regional dopamine release. Nature Communications. nature.com. https://doi.org/10.1038/s41467-018-08143-4
Mahadi, K. M., Lall, V. K., Deuchars, S. A., & Deuchars, J. (2019). Cardiovascular autonomic effects of transcutaneous auricular nerve stimulation via the tragus in the rat involve spinal cervical sensory afferent pathways. Brain Stimulation, 12(5), 1151–1158. https://doi.org/10.1016/j.brs.2019.05.002
Bravo-Hernandez, M., Tadokoro, T., Navarro, M. R., Platoshyn, O., Kobayashi, Y., Marsala, S., … Marsala, M. (2020). Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nature Medicine, 26(1), 118–130. https://doi.org/10.1038/s41591-019-0674-1
Zhang, S. B., Lin, S. Y., Liu, M., Liu, C. C., Ding, H. H., Sun, Y., … Xin, W. J. (2019). CircAnks1a in the spinal cord regulates hypersensitivity in a rodent model of neuropathic pain. Nature Communications. nature.com. https://doi.org/10.1038/s41467-019-12049-0
Koffler, J., Zhu, W., Qu, X., Platoshyn, O., Dulin, J. N., Brock, J., … Tuszynski, M. H. (2019). Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nature Medicine, 25(2), 263–269. https://doi.org/10.1038/s41591-018-0296-z
ACCESSORIES
Supplied
- Operator’s Manual
- One set of batteries (fitted)
- One Pair of output plugs (NL985P)
Recommended
- Additional NL985P Output Plugs
FAQS
The unit can be triggered fast enough to allow >99% “on” time, but the “off” time must >20 microseconds – so, very fast!
The DS3 does not have medical device directive (MDD) certification and has not been designed for safe human use. We offer a range of MDD compliant stimulators for human/patient connection.
Yes and no. The DS3 features an external “gate” input allowing externally derived trigger pulses to define stimulus duration. However, the output amplitude is always determined by the front panel setting of the stimulator. We recommend our NeuroLog system to those interested in using a computer generated waveform to define stimulus timing/polarity and strength.
Yes, there are two simple methods.
(i) The DS3 has the ability to be gated by an external pulse i.e. when external control of pulse duration is selected the output stimulus timing will be controlled by the trigger-in stimulus pulse. It should be noted that (with a 5V input pulse) there is only a very short delay of <2µs for the turn-on and <6µs for the turn-off. When a typical TTL pulse is used, which will only be about 3V, these are matched at 4µs each.
(ii) Alternatively, if a trigger pulse is not available or exact timing is not crucial (for instance when lesioning or injecting dye, there is a jumper which can be set to allow the unit to deliver a current for the duration the single shot button is held down. The internal “Single” jumper determines the output delivered when the “SINGLE” button is pressed (when “EXTERNAL” Duration is selected).
“MON” (factory setting) produces a very short pulse ~10µs when pressed.
“CONT” produces an active output for the duration of the press. This could be useful in dye marking etc.
The visible difference between the two units (apart from the colour!) is that the output control of the DS2A is defined in Volts while the output of the DS3 is defined in Amps. The actual stimulus passing through your preparation is measured in Amps in both cases and is dependent upon Ohms Law (V = IR). If your preparation has a variable impedance (R) and you are using a constant voltage (V) source such as the DS2A, then the actual current (I) passing through the tissue may vary considerably between each stimulus, which may not be a good idea if you want to apply reproducible stimuli. With the DS3, the constant current circuitry prevents variations in tissue impedance from altering the size of current applied (within the 90V compliance limit of the unit), leading to the stimulator equivalent of WYSIWYG – “What You Set Is What You Get”. Unfortunately there are no well defined rules governing the circumstances under which either stimulator should be used. If you are not sure and feel the need to evaluate either or both please contact us.
No, Digitimer no longer offers this service. Since the introduction of the new DS3 constant current stimulator, this unit is now offered to customers requiring a constant current version of the DS2A.
Yes, this is quite simple to setup and has been summarized in an application note.
Battery life will depend on the frequency of operation and will therefore vary widely with the application, but the following example may be considered typical. Output current – 20mA, duty cycle – 1:10, period of operation – 2 hours/day. Estimated life to end point voltage of 6V per 9V battery – 2 months. In in vitro electrophysiology applications, the battery life will most likely be far longer as the currents being used will be lower. Replacement batteries should be of premium grade sealed type, alkaline preferred and batteries should not be left in an unused stimulator.
Rechargeable batteries are not recommended, as their voltages tend to decline very suddenly, which could lead to a failure in the middle of an experiment.